What is PGT-A test?
PGT-A is a genetic test performed on embryos to identify numerical chromosomal abnormalities (aneuploidy).
Our technology, Next-Generation Sequencing (NSG), allows us to analyze all 24 chromosomes. Chromosomal abnormalities are detected prior to embryo transfer to enable ranking of embryos for transfer and increase pregnancy rates.
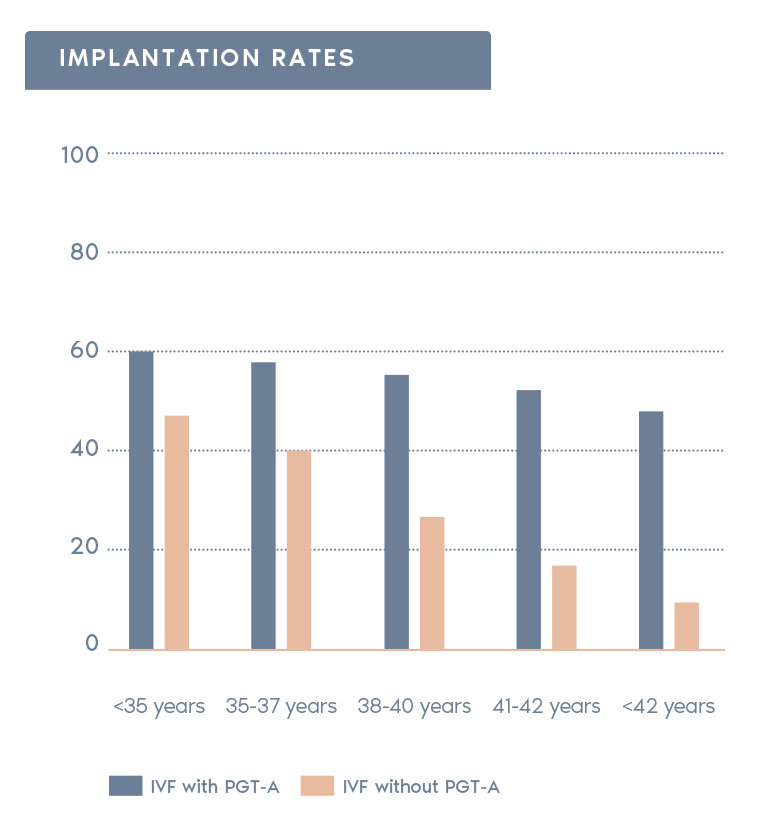
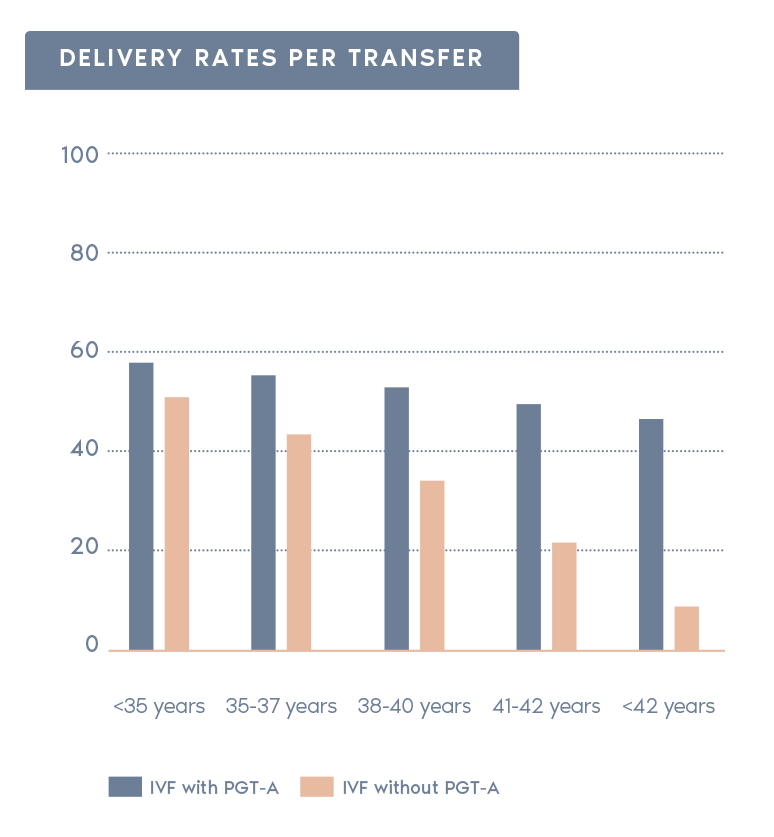
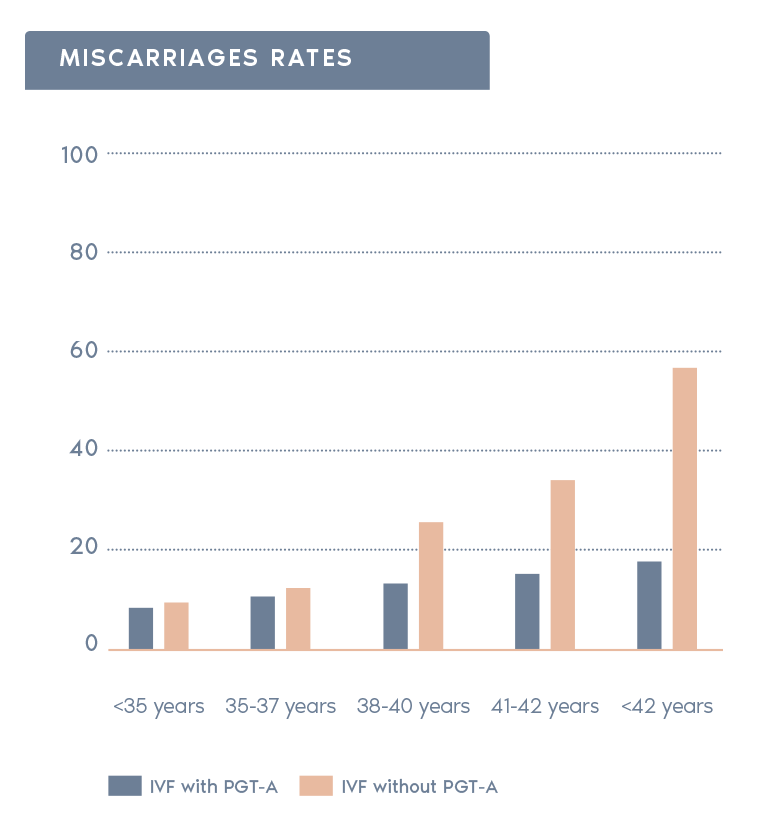
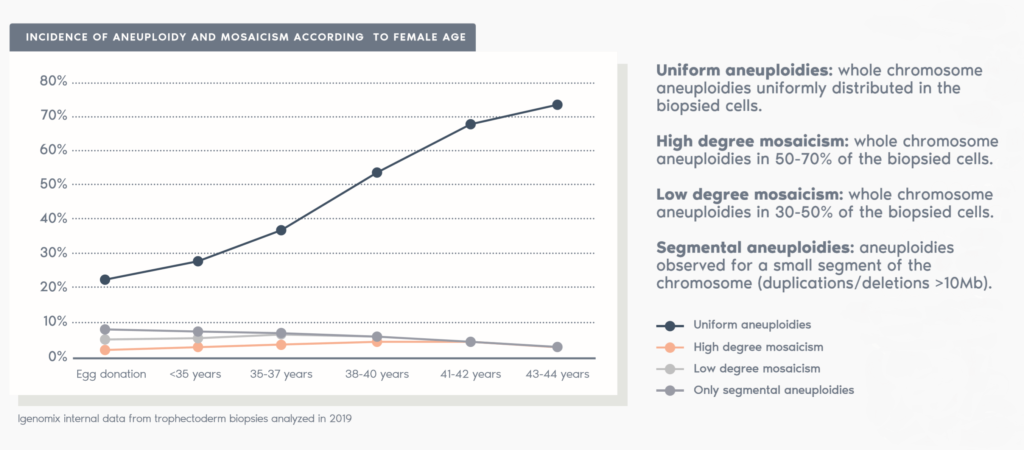
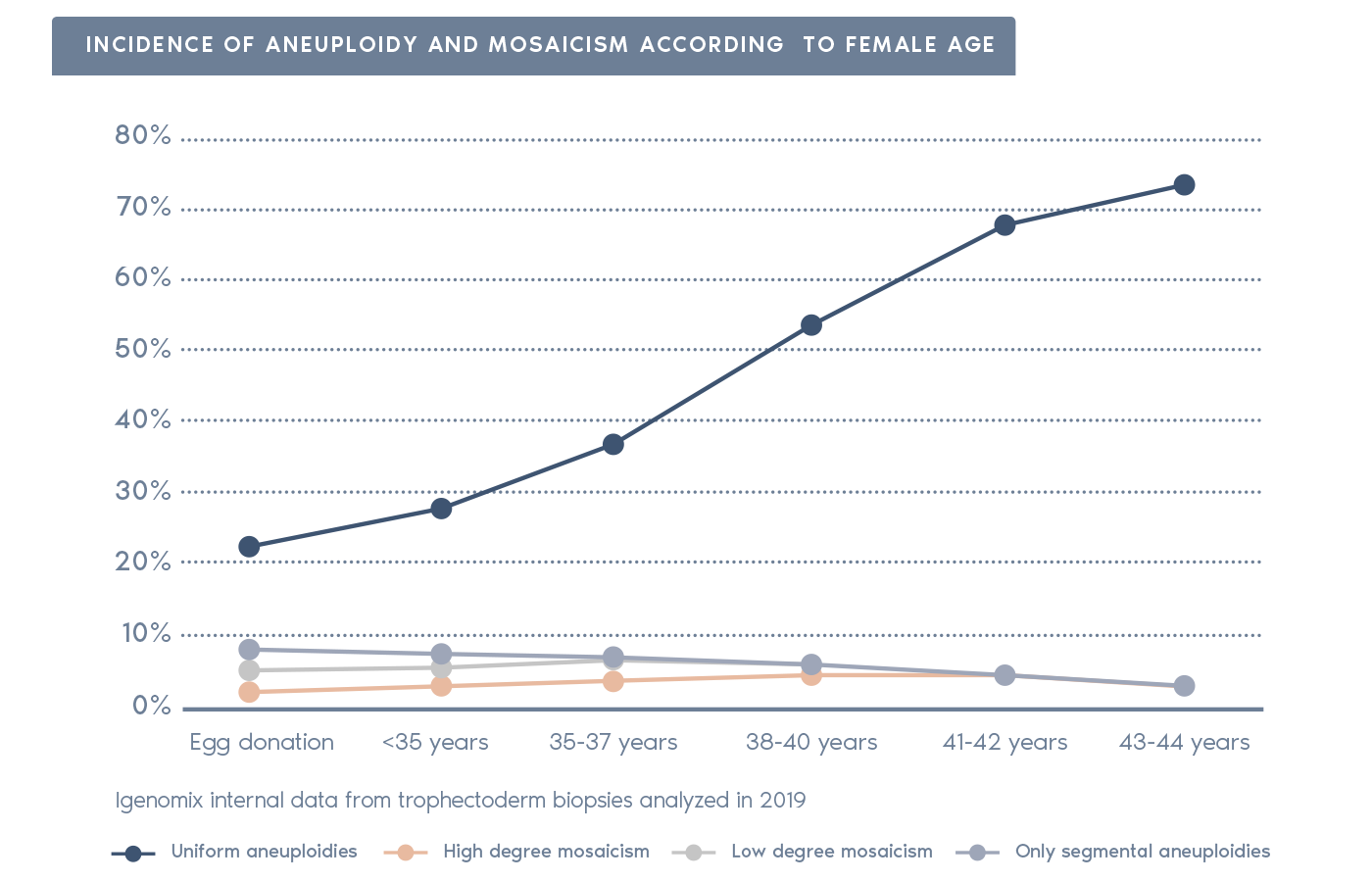
Clinical Outcome with and without PGT-A based on SART 2016 public database
SART 2016 (https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?reportingYear=2016)
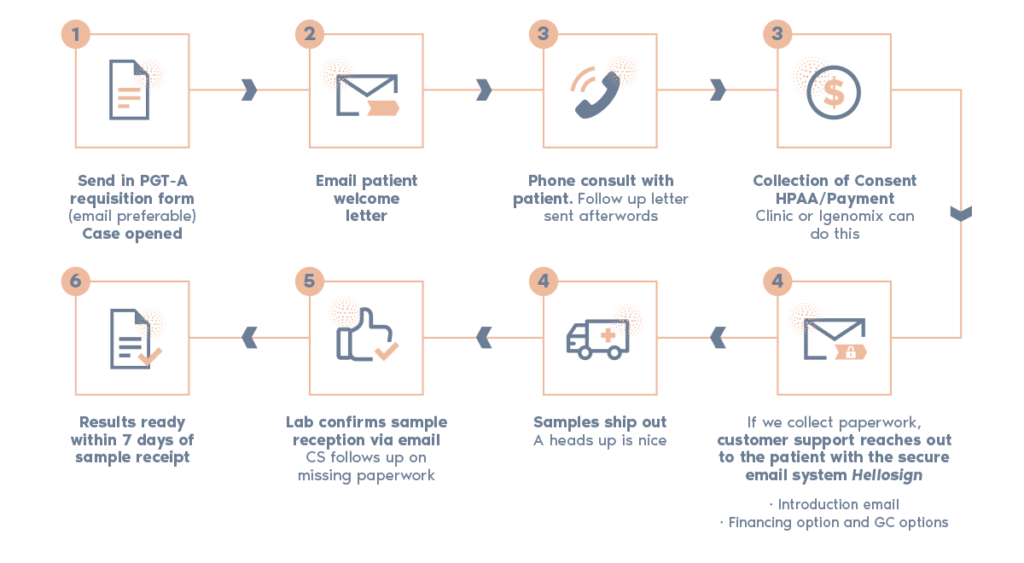
What is the procedure?
- Increases pregnancy rates per transfer:
Selecting chromosomally normal embryos can increase the rate of pregnancy after transfer.
- Reduction in miscarriage rate:
In the general population, 25% of all clinical pregnancies end in miscarriage. The risk for miscarriage is reduced if a euploid embryo is transferred.
- Increase in the likelihood of having a healthy baby:
Some pregnancies with aneuploidies can result in babies affected with chromosomal syndromes.
- Reduction in time and necessary resources:
The time and resources necessary to achieve a pregnancy are reduced.
- Reduces risk of multiple pregnancy:
Patient can utilize a single embryo transfer of a euploid embryo rather than transferring multiple untested embryos to reduce the risk of multiple pregnancy.
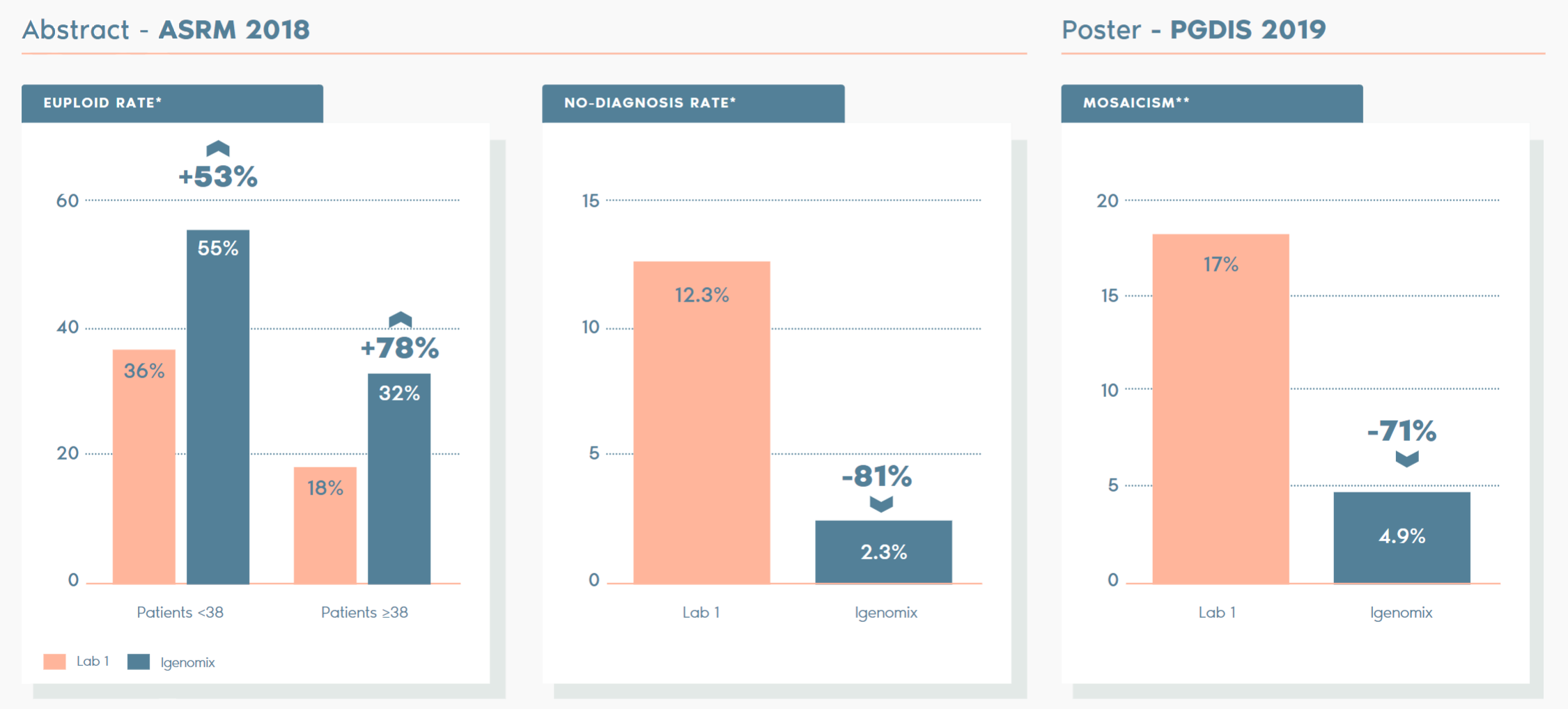
Why Igenomix PGT-A?
Independent studies have compared our PTG-A vs other labs and the key facts are as follows:
*ABSTRACT – ASRM 2018: A comparison of diagnostic results of Preimplantation Genetic Testing for Aneuploidy (PGT-A) from reference laboratories during a period of transition; trends and inherences for patient care. D. Ioannou, M. D. Baker, S. D. Jones, l. R. Grass, K. A. Miller. Embryology, IVF Florida Reproductive Associates, Margate, FL.
**POSTER – PGDIS 2019: Clinical comparison of two pgt-a PLATFORMS UTILIZING DIFFERENT THRESHOLDS TO DETERMINE PLOIDY STATUS. D. Monahan, G. Harton, D. Griffin, M. Angle, C. Smikle. Laurel Fertility Care, San Francisco, CA.
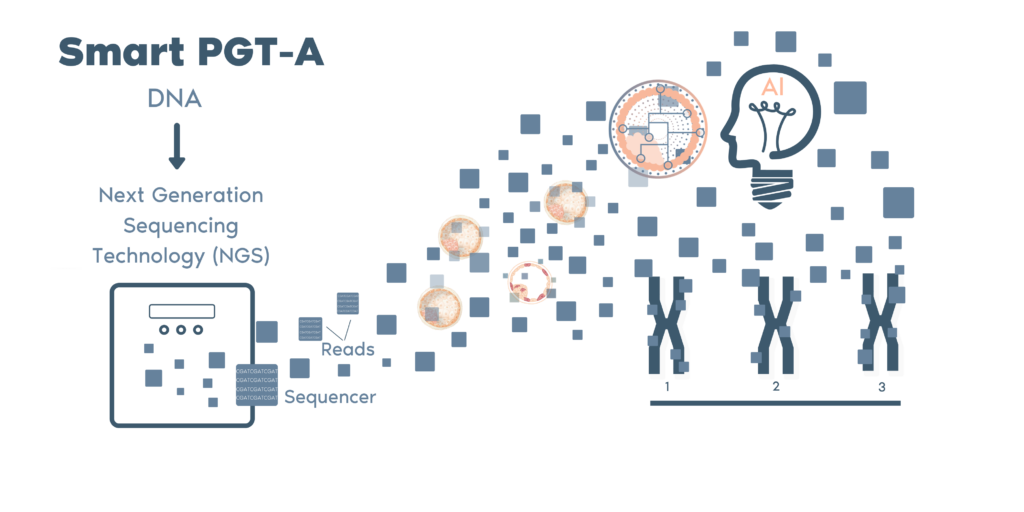
By applying the unique Smart PGT-A technology, Igenomix is able to constantly refine and improve our ability to analyze embryo samples. Wehave developed a proprietary bioinformatic calling algorithm based on over 100,000 embryo samples which minimizes human errors and bias/subjectivity.
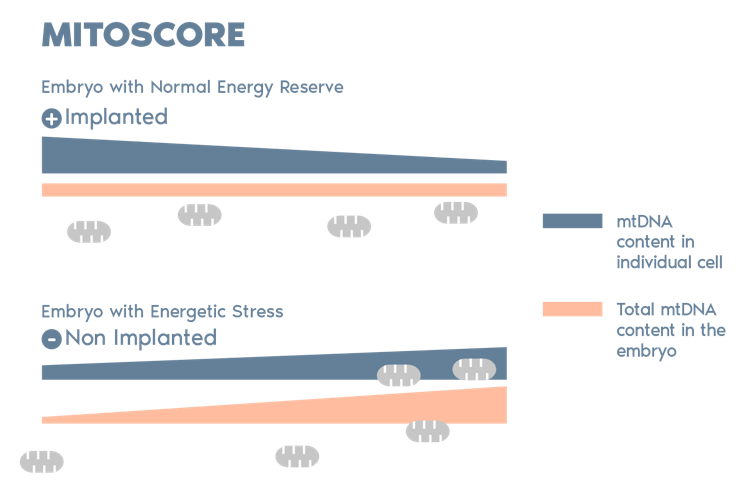
MitoScore is a mitochondrial biomarker developed by Igenomix which gives us an indicator of the energy status of an embryo.
MitoScore allows us to identify embryos with the greatest probabilities for implantation, and a viable pregnancy through IVF/ PGT-A.*
- Most DNA is in chromosomes within the nucleus, but mitochondria have their own DNA. This genetic material is known as mitochondrial DNA or mtDNA.
- mtDNA in an embryo is an index of energetic stress, which can be used to predict its implantation potential.
- Our studies indicate that an increase in the mitochondrial DNA in the embryo is indicative of an insufficient level of energy and a low implantation potential.